If the FDA approves aducanumab Biogen could go to circa 400 and if not to circa 200 Mizuho Securities analyst Salim Syed said in a recent research note. Which could mean booster shots would be recommended down the road.
Pfizer CEO says a COVID-19 vaccine booster or 3rd dose likely within 12 months.

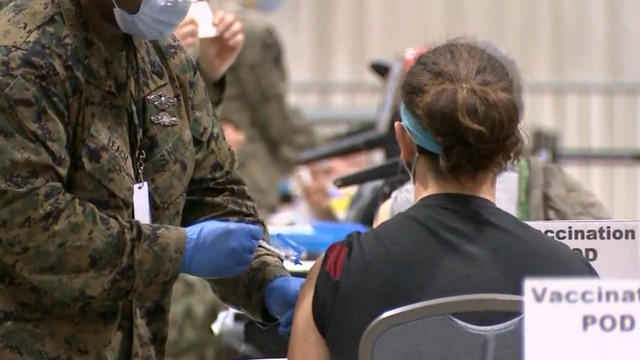
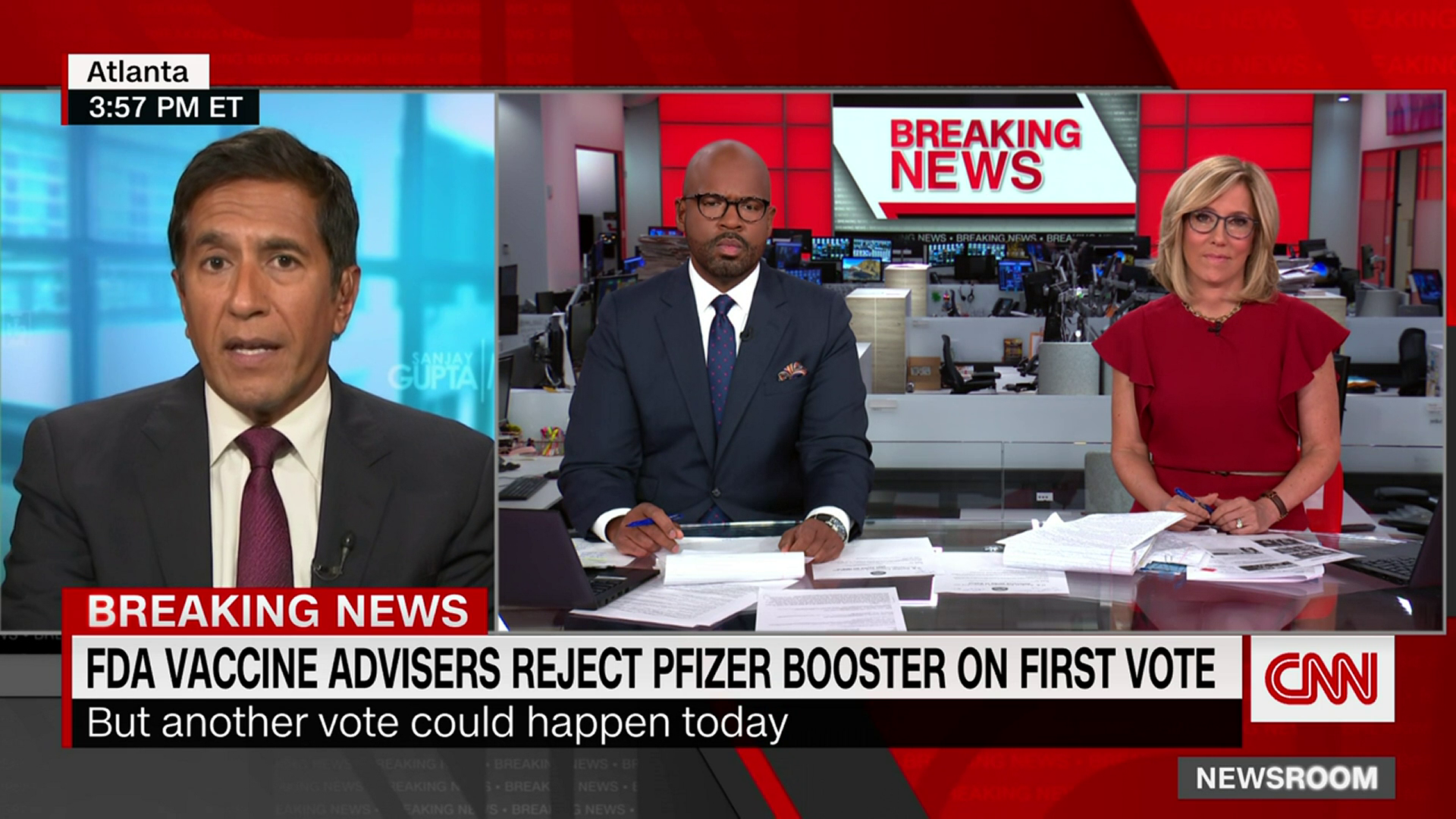
Fda booster vote. Doran Fink MD PhD deputy director of the FDAs Division of Vaccines and. McClellan the former FDA commissioner said the United States excess supplies of the shot exist in case of complications such as emerging variants or demand for a booster shot and that. Sanjay Gupta reports on these booster studies.
ET May 12 2021 CDC has reports of fewer than 10000 breakthrough Covid-19 infections in vaccinated people. Food and Drug Administration FDA expanded the emergency use authorization for the Pfizer-BioNTech COVID-19 Vaccine for the prevention of COVID-19 to include adolescents 12 through 15 years of age. 11 new deaths in Louisiana reported from Ida raising states death toll to.
June 24 2021 -- The US. Story continues Biogen shares were trading near 271 on Thursday down from their 52-week high of 35563 reached in November before the advisory committee vote. Americas Largest Teachers Union to Vote on Mandatory COVID Vaccinations Masks and Testing For Students.
The drugmaker says vaccine efficacy was 100 in its Phase 3 clinical trials. By edhat staff Today the US. Vote 2020 Texas.
Vaccine makers are preparing for a next possible phase of the Covid-19 vaccine rollout. Russian President Vladimir Putin self-isolating due to coronavirus among inner circle AP. The Washington DC City Council on Tuesday voted 8-5 to approve a bill to ban flavored tobacco products including those containing menthol.
If the FDA approves aducanumab Biogen could go to circa 400 and if not to circa 200 Mizuho Securities analyst Salim Syed said in a recent research note. FDA liaison representative Doran Fink MD PhD noted the agency will add a warning about the risk of myocarditis or pericarditis following vaccination that states these events have occurred in. Food and Drug Administration FDA is adding a warning to the fact sheets for the PfizerBioNTech and Moderna mRNA COVID-19 vaccines as medical experts continue to.
World Health Organization chief urges halt to booster shots for rest of the year By KEVIN McGILL and MELINDA DESLATTE. EL PASO Texas KVIA As more Americans get the Covid-19 vaccine the race to research booster shots continues. Moderna Pfizer and Johnson Johnson are all researching the potential use of.
The US Food and Drug Administration FDA is adding a warning to the fact sheets for the PfizerBioNTech and Moderna mRNA COVID-19 vaccines as medical experts continue to investigate cases of heart inflammation which are rare but are more likely to occur in young men and teen boys. Biogens aducanumab for Alzheimers The FDA is facing a no-win decision on Biogens Alzheimers treatment aducanumab. The National Education Association Americas largest teachers union is holding a vote on requiring mandatory COVID-19 vaccinations masks and testing for students before classes return in the fall.
Michele Cohen Marill June 23 2021. From CNNs Maggie Fox. But a CDC advisory panel where the FDA made the announcement still strongly backs the vaccines benefits which outweigh the rare risk for heart inflammation.
The FDA amended the emergency use authorization originally issued on Dec. Former Minneapolis police officers accused of violating George Floyds rights to be arraigned AP. Biogen shares were trading near 271 on Thursday down from their 52-week high of 35563 reached in November before the advisory committee vote.
Even then however there may only be certain people who need booster shots such as those over. 11 2020 for administration in individuals 16 years of age and older. Pfizer said all of the subjects are being randomized into three groups one with the 20vPnC plus Pfizer-BioNTech COVID-19 vaccine booster which is a third dose of the Pfizer-BioNTech COVID-19.
After collaborating with the company on review of its approval application in spite of mixed and controversial data a panel of outside advisers voted. In early April Pfizer and its partner BioNTech filed a request with the FDA to amend the emergency use authorization EUA to lower the eligibility age to 12.
Fda Panel To Discuss Booster Shots Today Here S What You Need To Know The Boston Globe
Fda Sounds Skeptical Note On Pfizer Booster Shot Ahead Of Key Vote Politico






/do0bihdskp9dy.cloudfront.net/09-17-2021/t_482efbcf87dd4f6fbc792baa8659bf7b_name_file_1280x720_2000_v3_1_.jpg)